
Effexor looks different depending on its dosage and release form. Effexor XR exists as extended-release capsules in 37.5mg (gray and peach), 75mg (peach), and 150mg (orange) strengths, each with distinctive imprints. Generic venlafaxine tablets come in immediate-release forms (25mg, 37.5mg, 50mg, 100mg) and extended-release tablets (37.5mg, 75mg, 150mg, 225mg).
The pills vary in color—white, peach, orange, yellow—and shape—round, oval, cylindrical. Manufacturers mark each pill with unique identifiers such as “W Effexor XR,” “OS 301,” or other imprint codes that indicate dosage strength. Proper identification assists patients taking this antidepressant medication correctly.
Table Of Contents:
What Does Effexor Look Like?
Most commonly it is used in the form of extended-release capsules, which are sold under the brand name Effexor XR. These capsules contain 37,5mg, 75mg, or 150mg of the active ingredient. Doctors may also prescribe venlafaxine tablets, which are sold under the generic name. These are immediate-release tablets with the following strengths: 25mg, 37,5mg, 50mg, 100mg. The same generic variant also has extended-release oral tablets, with the strengths: 37,5mg, 75mg, 150mg, and 225mg.
Below, some common venlafaxine capsules and tablets are described.
Effexor Extended-Release Capsules
Effexor capsules have a cylindrical shape, made from dissoluble gelatin, which contains venlafaxine powder. Capsules cannot be divided or cut into pieces.
Gray and Peach 37.5 mg Capsules
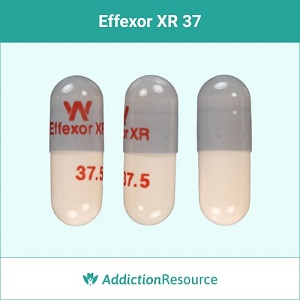
37.5 mg if the lowest dose of the branded drug available. The capsule is grey at the top, and the lower part is of peach color. The imprint on the upper part-reads W Effexor XR. The lower part has 37.5 imprinted, which indicates the dosage. Pfizer manufactures this type of capsules.
Peach Effexor XR 75 mg Capsules
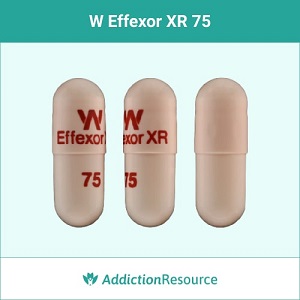
This is a peach capsule is 75mg in strength. Venlafaxine ER 75mg capsules present the usual starting dose for most patients with generalized anxiety disorder. The imprint is printed in red with X Effexor XR on the upper part and 75 on the lower. It belongs to the group of SNRI (Serotonin-norepinephrine reuptake inhibitors) antidepressants.
Orange W Effexor XR 150 Capsules

The strength of such capsules is venlafaxine 150 mg, which is a high dose. This capsule is red and has W Effexor XR imprinted on the upper part.
Venlafaxine Tablets Images
Tablets are essentially compressed powder and can take on a lot of shapes and colors:
Gray OS 301 (ER 37.5 mg)

This is an extended-release formulation containing venlafaxine 37.5 mg. The pill is white and round. It has an imprint of OS and 301 below it. Upstate Pharma LLC supplies this type of tablet. Effexor side effects include constipation, insomnia, dry mouth, and fatigue.
Gray OS 302 (ER 75 mg)

OS 302 tablet is also supplied by Upstate Pharma LLC and has the same color and shape. Venlafaxine 75 mg is a mild dose of the medication.
Gray OS 303 (Venlafaxine ER 150 mg)

This formulation contains venlafaxine er 150 mg, which is a strong dose. Withdrawal symptoms after taking such an amount long-term will be more pronounced. Therefore it is advised not to try to get off the medication without a doctor’s assistance.
Gray OS 304 (ER 225 mg)

OS 304 pill contains 225 mg of the active ingredient. This is the strongest dose available, and it is not available in the form of Effexor capsules.
Gray and Peach W 716
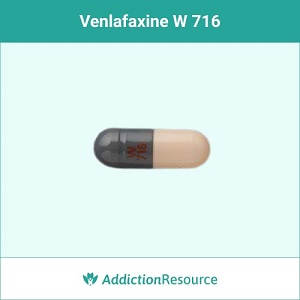
The gray & white capsule with a red imprint W 716 contains 37.5 mg of the active ingredient. Effexor can cause weight gain or loss. It’s advised to consult a doctor if such side effects disturb the patient.
Peach W 717
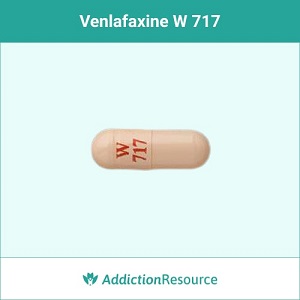
The capsule with a red imprint W 717 on the upper part is identified as a venlafaxine 75 mg capsule. It is an extended-release formulation supplied by Wockhardt USA LLC.
Orange W 718

This capsule contains venlafaxine 150 mg, and is also an extended-release formulation. It is orange and has the letter W and number 718 imprinted. It is not recommended to combine Effexor and alcohol, because it can lead to the development of more adverse reactions. Moreover, alcohol potentiates symptoms of overdose, which can lead to increased lethality in such cases.
Gray and White ZA-35 Capsule

The strength of this type of capsule is identified by the imprint on the lower part, which is 37.5 mg. The imprint on the upper part reads ZA-35. It is an extended-release formulation of the generic drug.
White Venlafaxine ZA-36

The color of the ZA-36 capsule is peach and white. It also has the dosage imprinted, being 75 mg. Inactive ingredients include sodium lauryl sulfate, ferrosoferric oxide, silicon dioxide, hypromelloses, cetostearyl alcohol, microcrystalline cellulose, magnesium silicate, gelatin, titanium dioxide, ferric oxide red.
Red and White ZA-37
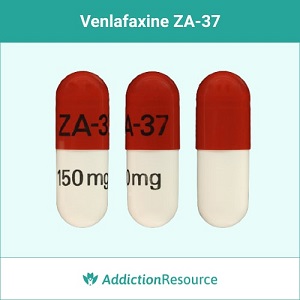
This capsule contains venlafaxine 150 mg. The upper part is red and has ZA-37 imprinted. The lower part is white with the strength imprinted.
Yellow 25 mg tablet (IR)

The pill with an imprint 175 on one side and a horizontal line on the other contains 25 mg of the active ingredient. It is identified as venlafaxine hydrochloride immediate release.
Pink 37.5 mg tablet (IR)
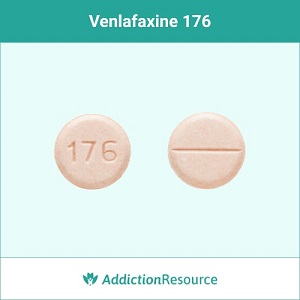
A round tablet with an imprint 176 is identified as venlafaxine 37.5 mg. The supplier is Northstar Rx LLC.
Venlafaxine White Oval IP 304 75 mg tablet (IR)

The imprint on venlafaxine 75 mg tablet is IP on the left side and the number 304 on the right. There is a vertical line between them. The tablet is oval.
Venlafaxine 100 mg I 22 tablet (IR)

I 22 pill is peach and round. There is a horizontal line under “I,” and the number 22 is imprinted on the other side. It contains 100 mg of the active ingredient, which is a strong dose.
Extended-Release vs. Immediate-Release
Oral tablets of the venlafaxine trade name may be available in immediate-release (IR), and extended-release (ER) formulations in the tablet form only. Immediate-release tablets release into the system as soon as you take them. In contrast, extended-release formulations are released gradually and act longer.
Venlafaxine ER 150 mg (or other strengths) is an extended-release drug formulation. Venlafaxine HCL ER has fewer side effects than the brand name; however, it has no advantage over immediate-release formulation in reducing Effexor withdrawal effects. Effexor half-life of the immediate-release formulation is five hours. In comparison, the extended-release formulation half-life is about 12 hours.
Effexor XR
Effexor XR is the sustained-released form of the substance. These capsules are only available as extended-release formulations. Effexor XR is characterized by extending the drug’s plasma concentration time (while immediate-release formulation usually takes about 4 to 6 weeks to appear its effects).
This formulation is assigned an AB rating by the FDA (meets the necessary bioequivalence standards established by the US Food and Drug Administration). In contrast, venlafaxine hydrochloride extended-release form isn’t.
The original formula of the drug was discontinued from the US market because it was required for the patient to take 2 to 3 doses daily, while Effexor XR can be taken once daily. Moreover, it causes less nausea than the original formula.
Storage of Effexor
Effexor tablets are usually stored in standard carton boxes. Larger amounts of capsules can also be found in plastic bottles, which is more convenient. All medicines should be kept in their original packages and stored at moderate room temperature (from 20 to 25 degrees C, or 68 to 77 degrees F) away from moisture and heat in a tightly sealed container. Avoid storing this medication in areas where it could get damp or wet, such as bathrooms.
How To Choose The Best Form?
Only a doctor can choose the best form of Effexor tablets or capsules for each patient. Still, the ones with graver mental health problems might find it more beneficial to use extended-release pills. One study has shown that this is more efficient than immediate-release formulation. Another study proved that ER pills have fewer risks than IR tablets. However, only a doctor can appropriately weigh the pros and cons of Effexor tablets vs. capsules. Moreover, only a professional can assess the contraindications and determine whether a patient should take these pills.
Various withdrawal symptoms may appear if one stops taking this medication without consulting with a doctor. People addicted to Effexor tablets can treat their condition with psychotherapy, support groups, and numerous other addiction treatment programs. There are drug rehab centers with diverse addiction programs across the states, which might be particularly efficient in helping people with addiction to this medication.
Hope Without Commitment
Find the best treatment options. Call our free and confidential helpline
Most private insurances accepted
Find Drug Rehabilitation Centers Near You Anywhere In the US
Addiction Resource team has compiled an extensive list of the top drug rehabilitation facilities around the country. Use our locator tool to find the best centers near you.
Page Sources
- Cunningham, L. A. (1997). Once-daily venlafaxine extended release (XR) and venlafaxine immediate release (IR) in outpatients with major depression. Annals of clinical psychiatry, 9(3), 157-164.
- Entsuah, R., & Chitra, R. (1997). A benefit-risk analysis of once-daily venlafaxine extended release (XR) and venlafaxine immediate release (IR) in outpatients with major depression. Psychopharmacology bulletin, 33(4), 671.
- DeVane, C. L. (2003). Immediate-release versus controlled-release formulations: pharmacokinetics of newer antidepressants in relation to nausea. The Journal of clinical psychiatry, 64(suppl 18), 14-19.


 Reviewed by:
Reviewed by:  Written by:
Written by: 
 FindTreatment.gov
FindTreatment.gov