
Desvenlafaxine, marketed under the brand name Pristiq, is a widely prescribed antidepressant medication used to treat major depressive disorder (MDD).
As an antidepressant that works by modulating brain chemistry, discontinuing Pristiq can lead to withdrawal symptoms due to the brain’s adaptation to the medication. With approximately half a million Americans under Desvenlafaxine treatment, patients and healthcare providers need to be up to date about the potential challenges of discontinuing this medication.
Continue reading for professional information about possible withdrawal symptoms, their timeline, and proper care for weaning off Pristiq.
Table Of Contents:
Pristiq Withdrawal Overview
Desvenlafaxine, the generic form of Pristiq, is an FDA-approved antidepressant that belongs to a category of drugs that inhibits the reuptake of both serotonin and norepinephrine, known as SNRIs.
It is approved for the treatment of major depressive disorder (MDD) in adults. Its off-label applications include treating hot flashes during menopause in healthy women who have contraindications to estrogen and major depressive disorder in treatment-resistant depression in adolescents.
Side effects or depression improvement may lead a patient to stop taking Pristiq. However, getting off Pristiq after long-term use can lead to withdrawal as the brain has adjusted to the higher levels of neurotransmitters mediated by the medication.
Other causes for withdrawal from Pristiq are :
- Dependence: Patients with a history of substance abuse may develop dependence.
- Tolerance: Patients may need higher doses after prolonged use, increasing withdrawal probability.
- Dose Reduction: Gradual dose reduction can still trigger withdrawal symptoms.
It’s safe to know that while Desvenlafaxine is not classified as addictive, it can cause physical dependence when the medication is stopped. This dependence shouldn’t be confused with addiction.
Pristiq Withdrawal Symptoms
Withdrawal symptoms of Pristiq can be grouped under the condition of antidepressant discontinuation syndrome (ADS), which is a group of emotional and physical symptoms that occur upon the abrupt cessation or decrease in dosage of antidepressants.
Surveys suggest half of patients experience withdrawal effects, with nearly half of them describing these effects as “severe.” Some patients report symptoms lasting for months or even a year after stopping antidepressants.
You can expect the following symptoms when tapering off Pristiq:
- Flu-like symptoms
- Dizziness
- Strange neural sensations, such as brain zaps or unexplained itching
- Nausea
- Vomiting
- Cramps
- Diarrhea
- Depression
- Anxiety
- Sleep issues
- Coordination impairment
Since all major classes of antidepressants can cause withdrawal symptoms when stopped or when the dose is reduced in a significant number of patients, caution is advised to avoid misdiagnosing withdrawal from Pristiq. The severity of these symptoms varies and is influenced by genetics, duration, frequency, and dosage. The longer you have used Pristiq, the higher the chance for you to experience withdrawal.
How Long Does Pristiq Withdrawal Last?
Taking notes about the potential Pristiq withdrawal timeline is crucial for discontinuation planning. The duration can vary significantly from person to person based on factors such as dosage, duration of use, and individual physiology. On average, symptoms occur within two to four days after drug cessation.
Below is a general chart that outlines a standard Pristiq withdrawal duration:
| Time Frame | Symptoms and Experience |
|---|---|
| 1-3 Days | Initial withdrawal symptoms may begin, including anxiety, irritability, and dizziness. |
| 1-2 Weeks | Symptoms often peak during this period. Common effects include headache, nausea, insomnia, and flu-like symptoms. |
| 2-4 Weeks | Symptoms gradually decrease in intensity. Some patients may still experience mood swings, brain fog, and fatigue. |
| 1-3 Months | Most acute withdrawal symptoms subside. However, some patients may continue to experience mild symptoms such as occasional dizziness and mood fluctuations. |
| +3-6 Months | Some individuals experience residual symptoms, including mild cognitive issues and mood disturbances. In rare cases, symptoms may last longer, requiring additional medical support. |
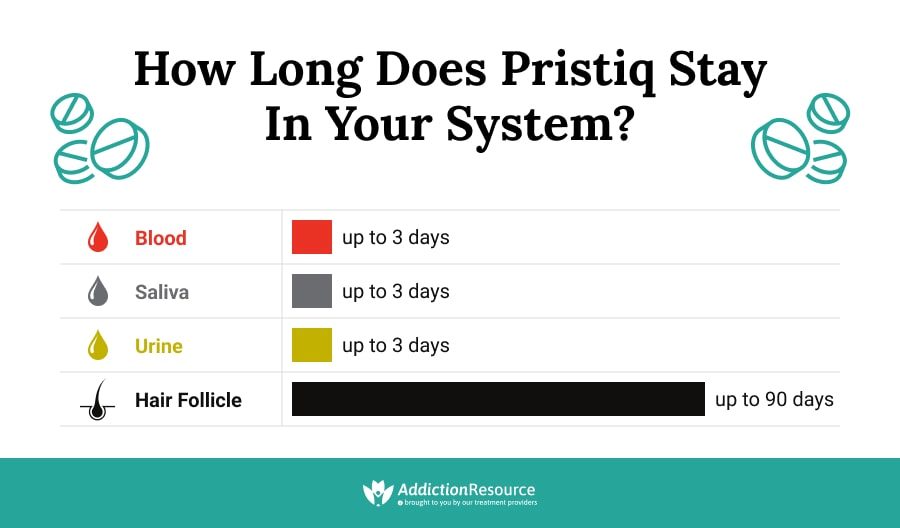
How Long Does Pristiq Stay In Your System?
It’s safe to remember that the start of the Pristiq withdrawal process is primarily influenced by the drug’s half-life, which is the time it takes for half of the drug to be eliminated from the body.
The Pristiq half life is approximately 11 hours, meaning it takes about 11 hours for the concentration of Pristiq in the blood to reduce by half. This relatively short half-life means that the drug leaves the system fairly quickly, impacting the onset and intensity of withdrawal symptoms. Desvenlafaxine can stay in the system for approximately 2.5 days; however, it can be detected in urine, blood, saliva, and hair for a longer time frame.
By knowing your antidepressant half-life, you can properly anticipate and manage desvenlafaxine withdrawal symptoms.
What Helps With Pristiq Withdrawal?
If you are considering stopping Pristiq medication, proceeding gradually and carefully is important. Going cold turkey with antidepressants may complicate and exacerbate the symptoms.
Here are vital steps to help manage withdrawal and receive proper care:
- Avoid rushing to stop taking antidepressants as soon as your symptoms improve.
- Discuss the benefits and risks of stopping antidepressants with your doctor.
- Avoid getting off Pristiq during stressful periods or significant life changes.
- Make a Pristiq taper off schedule with your provider. Aim for reductions between two to six weeks.
- Keep a mood calendar to track your daily mood on a scale of one to ten.
- Consider psychotherapy to manage emotional symptoms and to reduce relapse risk.
- Stress reduction techniques, regular sleep, and exercise can help prevent relapse.
- Stay in touch with your provider during the process. Report any physical or emotional symptoms.
- Ongoing check-ins are great tools to ensure you are feeling well and supported.
Tapering may not be necessary if you have been taking an antidepressant for less than four weeks or are taking fluoxetine. If you are on a low dose of Pristiq, tapering might be faster.
However, gradual tapering may not always prevent withdrawal syndrome. If you experience severe symptoms, reintroducing Pristiq and starting a slower tapering process may be helpful to reduce Pristiq withdrawal symptoms.
Pristiq Withdrawal − Bottom Line
Stopping Pristiq requires a prearranged and gradual approach. Planning a Pristiq taper schedule with your provider ensures a safe transition to be antidepressant-free.
Remember that while a desvenlafaxine tapering schedule can minimize withdrawal effects, it may not fully eliminate them. If you experience severe symptoms, a slower tapering or reintroducing of the medication might be necessary. Every individual’s experience with withdrawal can look different, and professional guidance will reassure you that you are taking the proper steps.
Pristiq addiction is not common, but it can cause dependence syndrome in vulnerable individuals, particularly those with a history of substance abuse. If you suspect Pristiq misuse, find a local rehab center for treatment and guidance.
People Also Ask
How to stop taking desvenlafaxine?
To discontinue desvenlafaxine, plan a personalized tapering plan with your healthcare provider to gradually reduce your dosage over several weeks to months. This approach minimizes potential withdrawal symptoms and ensures a safer transition off the medication.
How to taper off Pristiq 50mg?
Tapering off Pristiq 50mg involves reducing the dose in small increments under the guidance of your healthcare provider. A typical tapering schedule may span several weeks, allowing your body time to adjust to each lower dose.
Can you die from Pristiq withdrawal?
While rare, severe Pristiq withdrawal symptoms can lead to complications. These may include severe mood changes, neurological effects and physical symptoms. Seek medical guidance if symptoms worsen over time.
Hope Without Commitment
Find the best treatment options. Call our free and confidential helpline
Most private insurances accepted
Find Drug Rehabilitation Centers Near You Anywhere In the US
Addiction Resource team has compiled an extensive list of the top drug rehabilitation facilities around the country. Use our locator tool to find the best centers near you.
Page Sources
- Bcps, S. P. K. P. (n.d.). Desvenlafaxine - Drug usage Statistics, ClinCalc DrugStats database.
- Naseeruddin, R., Rosani, A., & Marwaha, R. (2023, July 10). Desvenlafaxine. StatPearls - NCBI Bookshelf.
- Horowitz, M. A., et al. (2023). Estimating Risk of Antidepressant Withdrawal from a Review of Published Data. CNS Drugs, 37(2), 143-157.
- Gabriel, M., & Sharma, V. (2017). Antidepressant discontinuation syndrome. CMAJ: Canadian Medical Association Journal, 189(21), E747.
- Moncrieff, J., Read, J., & Horowitz, M. A. (2024). The nature and impact of antidepressant withdrawal symptoms and proposal of the Discriminatory Antidepressant Withdrawal Symptoms Scale (DAWSS). Journal of Affective Disorders Reports, 16, 100765.
- Hodding, G. C., Jann, M., & Ackerman, I. P. (1980). Drug Withdrawal Syndromes: A Literature Review. Western Journal of Medicine, 133(5), 383-391.
- Liebowitz, M. R., & Tourian, K. A. (2010). Efficacy, Safety, and Tolerability of Desvenlafaxine 50 mg/d for the Treatment of Major Depressive Disorder:A Systematic Review of Clinical Trials. Primary Care Companion to The Journal of Clinical Psychiatry, 12(3).
- Harvard Health. (2022, May 15). Going off antidepressants.


 Reviewed by:
Reviewed by:  Written by:
Written by: 
 FindTreatment.gov
FindTreatment.gov